Alzheimer’s disease (AD), characterized by abnormal brain accumulation of amyloid-beta (Aβ) peptides, is the most common cause of dementia with limited cure. Recent study has recognized cerebrovascular dysfunction as an early event and a major contributor to disease progression. Understanding the basic pathogenesis of cerebrovascular dysfunction could aid in identification of novel drug targets for AD diagnosis and treatments. Epidemiological and pathophysiological evidence have supported a strong association between AD and type 2 diabetes mellitus (T2DM), both of which manifest insulin resistance in the blood brain barrier (BBB) endothelium that lines the cerebrovascular lumen. Published reports have shown that insulin signaling pathway at the BBB endothelium regulates Aβ trafficking and accumulation at the BBB. However, the underlying molecular pathways of Aβ trafficking and how they are disrupted in AD/T2DM are not well characterized. On the other hand, enhanced accumulation of Aβ within the BBB endothelium is suggested to augment BBB dysfunction by further increasing endothelial insulin resistance. Since BBB insulin signaling regulates several critical cerebrovascular functions, insulin resistance could aggravate cerebrovascular pathologies prevalent in 90% of AD patients. However, the synergistic effect of Aβ exposure and disrupted insulin signaling on BBB dysfunctions during AD and T2DM remains poorly understood.
PhD candidate Zengtao Wang (Pharmaceutics) worked on a project called Pharmacokinetics of Amyloid-β Protein Accumulation in the Blood-Brain Barrier Endothelium and Its Role in Cerebrovascular Dysfunctions in Alzheimer’s Disease as a UMII MnDRIVE PhD Graduate Assistant. Since it is widely recognized that insulin resistance is associated with cerebrovascular disease that aggravates Alzheimer’s pathology, the goal of this research was to establish causal connections between insulin resistance, BBB dysfunction, and AD pathogenesis. Such discoveries will advance the understanding of AD pathogenesis and identify novel therapeutic targets.
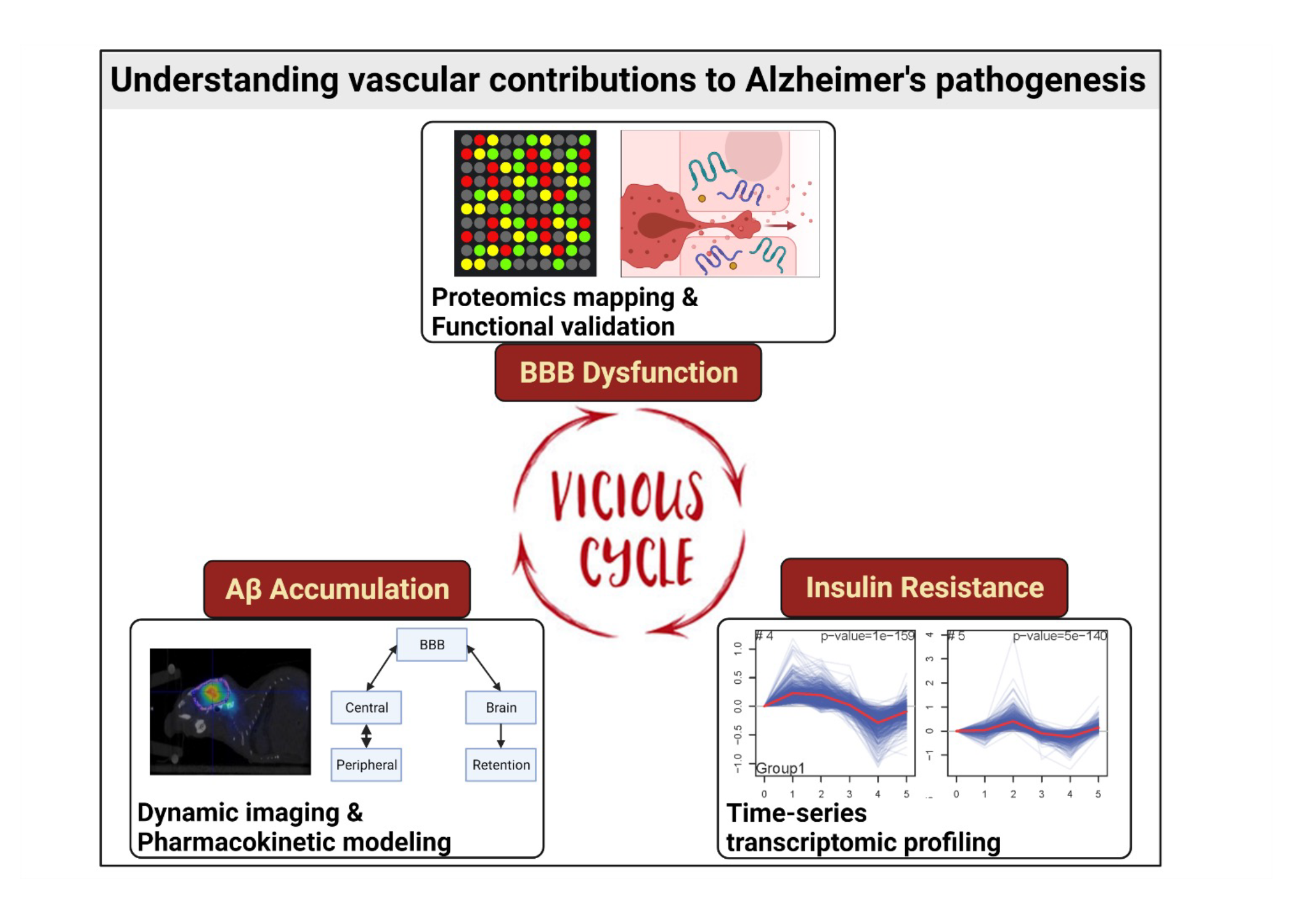
Results from the project include:
- Quantitative determination of Aβ40 and Aβ42 trafficking and accumulation kinetics at the BBB. Dynamic SPECT/CT imaging and pharmacokinetic (PK) modeling were employed to assess bi-directional transport of different Aβ isoforms and quantify their accumulations in the BBB. To facilitate imaging-based analysis for evaluating transport kinetics of macromolecules, a PK model was developed to deconvolve plasma PK of various radiolabeled tracers from their dynamic heart imaging data.
- Characterization of molecular mechanisms governing endocytosis and intracellular trafficking of Aβ at the BBB. Cellular uptake studies were performed under different endocytic perturbations using chemical inhibitors and siRNA knock-downs. Molecular imaging techniques have been used to track Aβ peptides in the endo-lysosomal system of BBB endothelium.
- Investigation of synergistic effect of Aβ exposure and insulin signaling in triggering BBB dysfunction. The dynamics of insulin-responsive pathways in BBB endothelial cells were mapped using time-series transcriptomic analysis. Inflammation related pathways were downregulated by insulin treatment and it was further found that insulin regulates inflammatory marker VCAM1 expression in a dose-dependent manner. A systems biology model was established and quantitatively simulated VCAM1 expression under disease conditions by integrating with proteomics and transcriptomics data.
Mr. Wang did this research under the supervision of Professor Karunya K. Kandimalla (Associate Dean, Pharmaceutics).
List of publications that have benefitted from the funding:
- Wang Z, Sharda N, Omtri RS, Li L, Kandimalla KK. “Amyloid-beta proteins 40 and 42 employ distinct molecular pathways for cell entry and intracellular transit at the BBB endothelium.” Mol Pharmacol. 2023 Aug 4:MOLPHARM-AR-2023-000670.
- Wang Z, Wang L, Ebbini M, Curran GL, Vernon CJ, Min HK, Siegel RA, Lowe VJ, Kandimalla KK. “Deconvolution of plasma pharmacokinetics from dynamic heart imaging data obtained by SPECT/CT imaging.” J Pharmacol Exp Ther. 2023 Jul;386(1):102-110.
- Wang Z, Tang X, Swaminathan SK, Kalari KR, Kandimalla KK “Mapping the Dynamics of Insulin-responsive Pathways in the Blood Brain Barrier Endothelium using Time-series Transcriptomics Data.” NPJ Syst Biol Appl. 2022 Aug 16;8(1):29.
Conference Presentations
Invited Presentations
- Wang Z, Tang X, Thompson KJ, Kalari KR, Kandimalla KK. “Systems biology modeling of the insulin signaling network in the blood-brain barrier endothelium predicts cerebrovascular inflammation in Alzheimer’s disease.” Special Poster Collection Curated by the AAPS President, AAPS Annual Meeting, Boston, MA, 2022
Wang Z, Wang L, Curran GL, Vernon CJ, Min PH, Lowe VJ, Kandimalla KK. “Prediction of plasma pharmacokinetics from dynamic heart imaging data.” Special Poster Collection Curated by the AAPS President, AAPS Annual Meeting, Philadelphia, PA, 2021
National Presentations
- Wang Z, Tang X, Thompson KJ, Kalari KR, Kandimalla KK. “Systems biology modeling of the insulin signaling network in the blood-brain barrier endothelium predicts cerebrovascular inflammation in Alzheimer’s disease.” Poster presentation, GPEN 2022, University of Minnesota, MN, 2022
- Wang Z, Veerareddy V (presenting author), Kandimalla KK. “Gut microbial metabolites regulate bidirectional transport and accumulation of Alzheimer’s disease amyloid beta peptides at the blood-brain barrier endothelium.” Poster presentation, AAPS Annual Meeting, Boston, MA, 2022
- Wang Z, Tang X, Swaminathan SK, Kalari KR, Kandimalla KK. “Mapping the dynamics of insulin-responsive pathways in the blood-brain barrier endothelium and impact of amyloid beta exposure.” Poster presentation, Barriers of the CNS Gordon Research Conference, New London, NH, 2022
- Wang Z, Kalari KR, Tang X, Swaminathan SK, Kandimalla KK. “Mapping the dynamics of insulin-responsive pathways in the blood-brain barrier endothelium using time-series transcriptomics data.” Poster presentation, AAPS Annual Meeting, Philadelphia, PA, 2021
The UMII MnDRIVE Graduate Assistantship program supported U of M PhD candidates pursuing research at the intersection of informatics and any of the five MnDRIVE areas:
- Robotics
- Global Food
- Environment
- Brain Conditions
- Cancer Clinical Trials
This project was part of the Brain Conditions MnDRIVE area.
The UMII program was converted to the Data Science Initiative-MnDRIVE Graduate Assistantship program in the fall of 2023. Research supported by the program is at the intersection of data science and the five MnDRIVE areas. The most recent Assistantships were announced in January 2024. The application period for the next awards will be announced in Fall 2024.