NK (natural killer) cells are a type of white blood cell. NK cell-based immunotherapies have been successful at treating some relapsed and/or refractory cancers. However, anti-tumor NK cells become dysfunctional in the tumor microenvironment, due in part to exhaustion, and this creates a barrier to durable and effective cancer treatments. Compared to the well-established molecular and genetic mechanisms controlling T cell exhaustion, there is limited knowledge of the processes that govern NK cell exhaustion, necessitating a robust and replicable model for probing the precise mechanisms controlling human NK cell exhaustion.
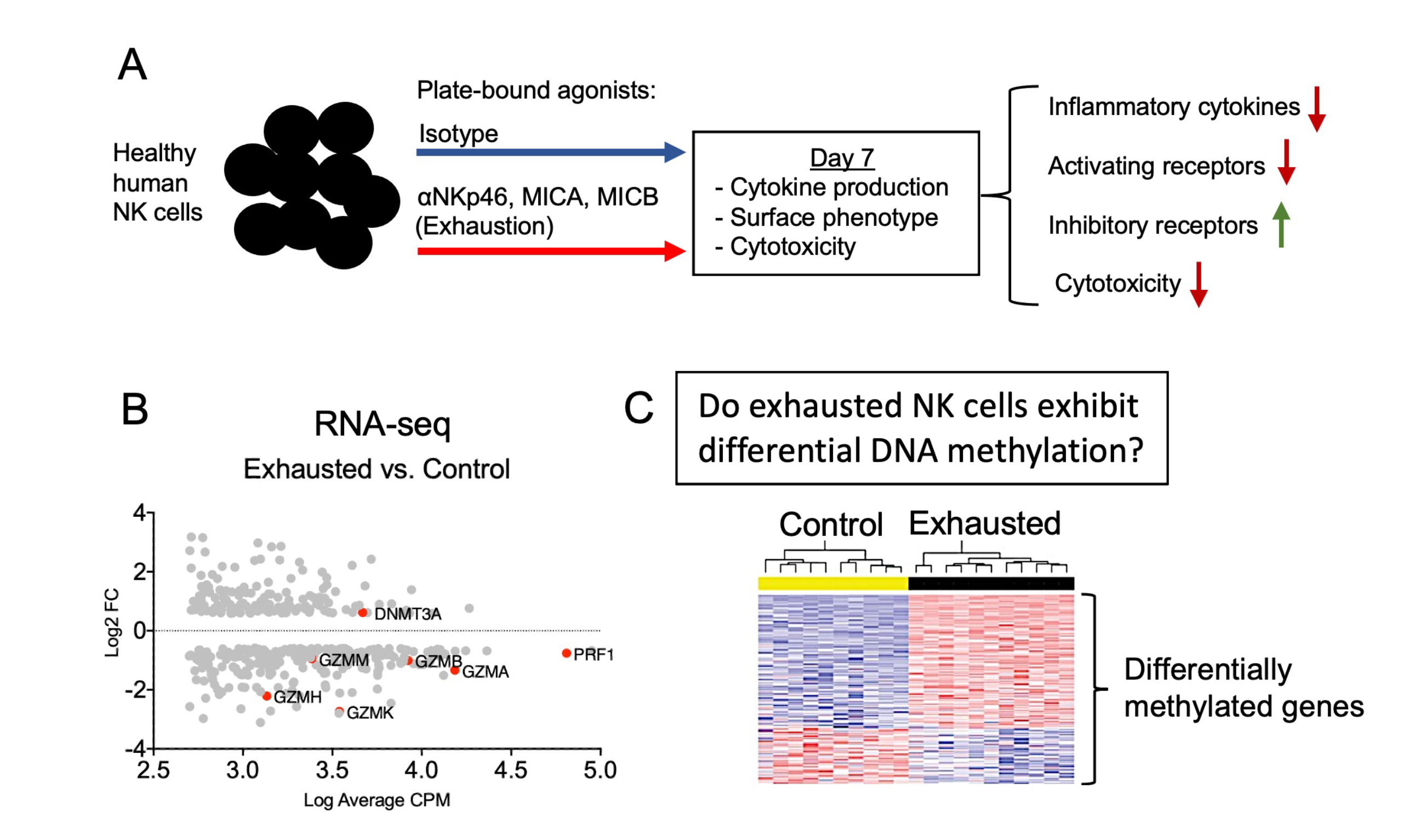
Professor Jeffrey Miller (Medicine; Masonic Cancer Center; MSI PI) and PhD student Jacob Myers are working on a project called “Understanding the Epigenetic Modifications of Exhausted NK Cells.” They have generated a novel model of exhaustion using plate-bound agonists of NKp46 and NKG2D, two activating receptors that mediate tumor cell killing. This model induces a dysfunctional state in NK cells that mirrors clinical NK cell exhaustion: upregulation of checkpoint markers, downregulation of activating receptors, abrogated cytokine production, and decreased cytotoxicity. RNA-seq analysis has demonstrated that key effector molecules required for tumor killing, like granzymes (GZM) and perforin (PRF1), are downregulated at the transcriptional level. These data offer a plausible explanation for the loss of cytotoxicity exhibited by exhausted NK cells. Despite these insights, the mechanisms underlying this transcriptional downregulation are unknown. One clue hinting towards a possible mechanism for this downregulation is the induction of a DNA methyltransferase DNMT3A that has been implicated in driving T cell exhaustion. To investigate whether exhausted NK cell transcript abundance is influenced by DNA methylation, this team has begun a DNA methylation array study with the U of M Genomics Center. They are seeking to determine whether there are differential DNA methylation patterns between unstimulated and exhausted NK cell that match the differential transcriptional profiles of these two populations.
This project recently received a UMII Seed Grant. UMII Seed Grant funds are intended to promote, catalyze, accelerate and advance UMN-based informatics research in areas related to the MnDRIVE initiative, so that UMN faculty and staff are well prepared to compete for longer term external funding opportunities. This Seed Grant falls under the Cancer Clinical Trials research area of the MnDRIVE initiative.
Image description: The research model generates exhausted NK cells using healthy human NK cells. Enriched NK cells are chronically stimulated through their activating receptors (i.e. NKp46 and NKG2D) to induce exhaustion. Exhaustion is characterized by loss of cytokine production and cytotoxicity. (B) RNA-seq analysis of exhausted NK cells demonstrates that key effector molecules (e.g. granzyme B and perforin) are downregulated at the transcriptional level. (C) This research project will cover DNA methylation array analysis. The researchers are seeking to determine whether the DNA methylation patterns of exhausted NK cells correlate with the transcriptional signatures previously observed.